How Does Track and Trace Technology Help Protect Pharmaceutical Companies?
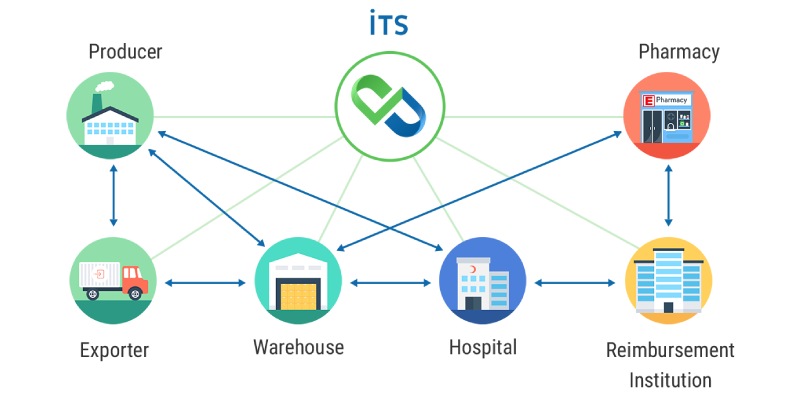
Track and trace technology is a sophisticated system designed to monitor and record the movement of products throughout the supply chain. Its primary purpose is to ensure transparency, accountability, and traceability from the manufacturer to the end consumer. By utilizing unique identifiers such as serial numbers, barcodes, or RFID tags, track and trace technology enables companies to monitor the entire lifecycle of a product, from production to distribution.
Grey Market Challenges for Pharmaceutical Companies
Pharmaceutical companies face unique challenges when it comes to the grey market. Counterfeit drugs and unauthorized distribution channels not only compromise patient safety but also undermine the integrity of the pharmaceutical industry. Moreover, grey market activities can result in significant financial losses for pharmaceutical companies, as they often disrupt established pricing strategies and erode brand value.
Role of Track and Trace in Grey Market Protection
Implementing track and trace technology plays a pivotal role in grey market solutions. Serialization and authentication mechanisms embedded within track and trace systems help prevent counterfeit drugs from entering the supply chain. By verifying the authenticity of products at each stage, pharmaceutical companies can ensure patient safety and maintain their reputation.
Furthermore, track and trace technology provides end-to-end traceability and transparency in the supply chain. It enables pharmaceutical companies to monitor the movement of their products, identify potential gaps or vulnerabilities, and take proactive measures to mitigate risks. This level of visibility helps prevent unauthorized distributors and parallel trade, ensuring that pharmaceutical products reach legitimate channels and authorized end consumers.
Implementing Track and Trace Technology in the Pharmaceutical Industry
The implementation of track and trace technology in the pharmaceutical industry is not without its challenges. Regulatory requirements and guidelines, such as those mandated by organizations like the Food and Drug Administration (FDA), must be adhered to. Pharmaceutical companies need to ensure compliance with serialization and reporting standards, which may require significant investments in infrastructure and systems.
Integration challenges are another aspect that pharmaceutical companies must address when implementing track and trace technology. Integrating track and trace systems with existing manufacturing, inventory management, and distribution processes can be complex. However, with proper planning, collaboration with technology providers, and employee training, these challenges can be overcome.
Success Stories of Track and Trace in Grey Market Prevention
Several pharmaceutical companies have successfully leveraged track and trace system pharmaceutical to combat grey market activities. For instance, Company XYZ implemented a comprehensive track and trace system across its supply chain, resulting in a significant reduction in counterfeit drugs reaching the market. This proactive approach not only protected patient safety but also safeguarded the company’s brand reputation and customer trust.
These success stories serve as compelling examples of how track and trace technology can be a game-changer in combating the grey market. By adopting best practices, pharmaceutical companies can mitigate the risks associated with the grey market and ensure the integrity of their products and supply chains.
In conclusion, the grey market poses substantial challenges to the pharmaceutical industry, threatening patient safety and company profitability. However, track and trace technology emerges as an ideal solution to address these issues. By implementing track and trace systems, pharmaceutical companies can prevent counterfeit drugs, ensure traceability and transparency, and detect unauthorized distributors. It is crucial for pharmaceutical companies to prioritize the adoption of track and trace technology to protect their brands, maintain regulatory compliance, and ultimately safeguard patient health. The future of combating grey market activities lies in the hands of proactive pharmaceutical companies willing to embrace this innovative technology.



